copper electron configuration|full electron configuration cu : Cebu The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write . Para proteger mejor la casa, habría que poner algo pegado a las puertas, desde una plataforma a modo de mesita (que puedes decorar con un jarrón o cualquier otra cosa de un solo bloque de ancho) hasta algo como una silla con una mesa de crafteo al lado, un sillón. cualquier cosa que evite que los zombies abran las puertas en una luna sangrienta.Teach with 51Talk | 51Talk
PH0 · longhand electron configuration for copper
PH1 · how to write electron configuration
PH2 · how many valence electrons does copper have
PH3 · full electron configuration cu
PH4 · elements that can expand valence when bonding
PH5 · electron configuration chart
PH6 · electron configuration calculator
PH7 · copper electron configuration full
PH8 · Iba pa
Russian Roulette de Rihanna, música para ouvir com letra, tradução e vídeo no Kboing. . tradução e vídeo no Kboing. Ouvir músicas. cadastre-se entrar. músicas; ao vivo; meu canal; top artistas; As mais ouvidas; playlists; estilos musicais; notícias; kboing fm; É+ FM; Página Inicial. Rihanna. Russian Roulette. Russian Roulette .
copper electron configuration*******The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. How to Write .In order to write the Calcium electron configuration we first need to know the .In order to write the Mg electron configuration we first need to know the .Potassium (K) - Electron Configuration for Copper (Cu, Cu+, Cu2+) - UMD
In order to write the Silicon electron configuration we first need to know the .Learn how copper atoms arrange their electrons across different shells and subshells, and how this affects its valency and chemical behavior. Find out the applications and . What is The Electron Configuration of Copper. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of Cu. If the general pattern of filling electron .
Writing Electron configuration of Copper. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for copper (Cu). You.
For example, the electron configurations of the transition metals chromium (Cr) and copper (Cu), are not those we would expect. Rather, Cr and Cu take on half . The electron configuration of copper is : 1s2 2s2 2p6 3s2 3p6 4s1 3d10. Cu has a unique fully-filled 3d configuration in its ground state and so has unique physical . Find the electron configuration of any element using this tool. Learn the rules, notation, and examples of electron configuration and valence electrons.
The electron configuration of copper is 1s2 2s2 2p6 3s2 3p6 4s2 3d9. Copper is a transition metal that is part of the copper family along with gold and silver. It has a . Copper is a chemical element with atomic number 29 which means there are 29 protons and 29 electrons in the atomic structure.The chemical symbol for Copper is Cu. Electron Configuration and . The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be .Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .From Sc on, the 3 d orbitals are actually lower in energy than the 4 s orbital, which means that electrons enter the 3 d orbitals first. In this video, we’ll discuss this in more depth and walk through all of the electron configurations for the 3 d transition metals. Created by Jay. Questions. In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.Electron configuration 3d 10 4s 1: Electrons per shell: 2, 8, 18, 1: Physical properties; . Copper is a chemical element; it has symbol Cu (from Latin cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with .copper electron configuration full electron configuration cu The electronic configuration of copper (Cu) can be represented as: 1s2 2s2 2p6 3s2 3p6 4s1 3d10. This configuration indicates that copper has 29 electrons distributed in its electron shells. The first shell has 2 electrons, the second shell has 8 electrons, the third shell has 18 electrons, and the fourth shell has 1 electron.
Let us use this smart electron configuration calculator to determine the electron configuration of copper: Choose copper (Cu) from the drop-down list of elements. Check the atomic number (29) and atomic mass (63.546) of copper. Read here how to calculate atomic mass. Read the full electron configuration: Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Copper (Cu) [Ar] 3d 10 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: 2, 8, 18, 1: 30: Electron configuration of Zinc (Zn) [Ar] 3d 10 4s 2: 1s 2 2s 2 2p 6 3s 2 .
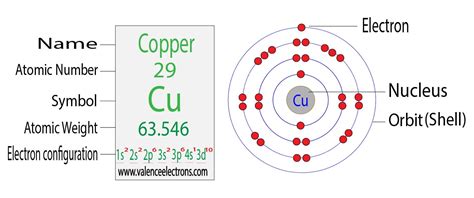
The electronic configuration of copper (Cu), with an atomic number of 29, is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d¹⁰. This unique configuration is characterized by one electron in the 4s orbital and ten electrons in the 3d orbital, which differs from the typical filling order. Copper’s 3d¹⁰ configuration in the third energy level (shell .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .

The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron .copper electron configurationElectron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Copper beads have been excavated in northern Iraq and which are more than ten thousand years old and presumably made from native copper, nuggets of which can sometimes .The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on). A single Copper atom has 29 protons and 29 electrons, but how do we know where Copper puts its electrons, in. Let's find the electron configuration of Copper!Similarly, the observed electron configuration of copper is [Ar]4s 1 3d 10 instead of [Ar]s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Figure 5.16.1 5.16. 1 Order of filling subshells in the building-up of atomic electron configurations. (a) Relative energies of subshells at the time they are being filled. (b) Aid to remembering the order of filling subshells. All possible shells having the same n value are written on horizontal lines. Diagonal arrows from lower right to upper .
The E-Gel Sample Loading Buffer is supplied as a ready to-use 1X solution, and is formulated specifically for maximum performance on E-Gel EX gels as well as other E-Gel precast gels. This buffer contains xylene cyanol FF and tartrazine dyes. Resources Nucleic acid electrophoresis products .
copper electron configuration|full electron configuration cu